Lumbar spinal stenosis (LSS) is a narrowing of the spinal canal in the lower back. Individuals who have LSS experience intense back pain and occasional weakness or numbness in their back and legs, and are often unable to walk for long periods of time.
How did I get LSS?
LSS is a naturally occurring condition caused by years of “wear and tear,” as well as bone degeneration that is a normal part of the aging process. Over time, the amount of space in the spinal canal becomes increasingly narrow, pinching the nerves in the lumbar spine and significantly reducing the nerves’ ability to exit to the lower extremities.
How can I tell if I have LSS?
The only way to be sure that your discomfort is a result of LSS is to consult with your physician. However, if you have experienced pain in your lower back and buttocks and weakness or numbness in your legs, these may be signs that you have developed spinal stenosis.
A New Option for LSS Patients
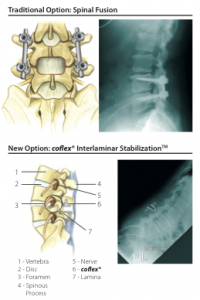
The coflex® Interlaminar StabilizationTM device is a new, non-fusion solution that can provide spinal stability — with greater mobility and faster recovery* — than spinal fusion surgery.
For decades, LSS patients’ surgical options were limited to either decompression or decompression with spinal fusion. In 2012, the FDA approved the coflex® spinal implant, which is a small, U-shaped titanium device that provides spinal stability without the mobility loss associated with spinal fusion.
How is the coflex® device implanted?
After the surgical decompression, which removes pressure on the impinged nerves, your surgeon will insert the coflex® implant through the same incision. The implant is then positioned onto the laminar bone, which is the strongest bone in the back of your spine.
The unique design of the coflex® implant maintains stability in the spine while preserving more natural movement at the affected area.
How do patients with coflex® compare to patients with spinal fusion?
A 2013 published study comparing the coflex® device with spinal fusion found 12 that the coflex® device had “advantages in perioperative outcomes,” and that “equivalent or superior 2-year clinical outcomes were seen with coflex®.” The 3 article concluded that “coflex® is a safe, efficacious, and viable alternative to spinal fusion in the treatment of spinal stenosis with low back pain.”
How long will I need to stay in the hospital or surgery center?
Three out of four coflex® patients in a clinical study left the hospital within 24–48 hours after surgery, compared to one out of three fusion patients.* For all coflex® patients in the clinical study, the hospital stay was less than a week. In some cases, the surgeon may elect to perform a decompression using the coflex® device at a surgery center, which means that some patients will not require a hospital stay.1
Will I need physical therapy?
When the surgeon says you can leave the hospital, the doctor may prescribe physical therapy. Always be sure to follow the physician’s instructions on physical rehabilitation and activities following surgery.
Will I need to take pain medication?
After surgery, medication may be provided by the surgeon. Based on the clinical study results, 85 out of 100 coflex® patients had significant pain relief at six weeks compared to 68 out of 100 patients who had fusion surgery.1
How soon can I resume activities of daily living after coflex® surgery?
The surgeon may ask you to return for an examination about six weeks after surgery.
The surgeon may also ask you to reduce your physical activities in the first six weeks after your operation. During the clinical study, walking during the first six weeks following surgery was usually acceptable. Please listen to your surgeon’s instructions on how much activity you can do after your surgery and for how long.
In the clinical study, patients were allowed to travel and engage in light activity such as walking as soon as they felt they could. It is important for you to realize that you have had a surgical operation. You should not participate in some activities until your surgeon has said you may do so. Please ask your surgeon when you can start doing certain activities. Your results may be different from patients in the clinical study.
Will my coflex® implant set off metal detectors?
The metal that makes up the coflex® implant may affect MR Imaging and metal detectors. You can talk to your surgeon about receiving a patient ID card. This card lets people know you have a coflex® implant in your back. You should show this card when you have X-Rays and MR Images. When you pass through an electronic detection system, you may use this card to tell security that you have this implant in your spine.
How do patients with coflex® compare to patients with fusion?
At two years, 88% of coflex® patients showed lasting relief of their spinal stenosis symptoms, compared to 78% of those undergoing spinal fusion.
What is adjacent segment disease and how does it affect spinal surgery?
Adjacent segment disease refers to degenerative changes in your intervertebral joints above and below the area of a spinal fusion or other back surgery caused by unphysiological motion based on fusion. It is a leading cause of patients requiring additional spinal surgeries over time.2-6 In the clinical study, coflex® patients retained their pre-operative range of motion at the areas below and above the treatment area.1